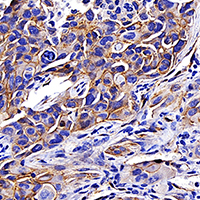
Tripartite motif-containing protein 6 facilitates growth and migration of breast cancer through degradation of STUB1
Submitted: 28 December 2020
Accepted: 2 February 2021
Published: 10 March 2021
Accepted: 2 February 2021
Abstract Views: 1120
PDF: 604
Supplementary: 123
HTML: 8
Supplementary: 123
HTML: 8
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Xin Kong, Qi Wang, Jie Li, Ming Li, Fusheng Deng, Chuanying Li, Mammaglobin, GATA-binding protein 3 (GATA3), and epithelial growth factor receptor (EGFR) expression in different breast cancer subtypes and their clinical significance , European Journal of Histochemistry: Vol. 66 No. 2 (2022)
- X.C. Xu, X. Abuduhadeer, W.B. Zhang, T. Li, H. Gao, Y.H. Wang, Knockdown of RAGE inhibits growth and invasion of gastric cancer cells , European Journal of Histochemistry: Vol. 57 No. 4 (2013)
- Yiming Zhang, Wanqiong Zheng, Liang Zhang, Yechun Gu, Lihe Zhu, Yingpeng Huang, LncRNA FBXO18-AS promotes gastric cancer progression by TGF-β1/Smad signaling , European Journal of Histochemistry: Vol. 67 No. 2 (2023)
- Xiangjun Lu, Jian Shen, Siyuan Huang, Dongdong Liu, Haitao Wang, Tumor cells-derived exosomal PD-L1 promotes the growth and invasion of lung cancer cells in vitro via mediating macrophages M2 polarization , European Journal of Histochemistry: Vol. 67 No. 3 (2023)
- Y. Zhang, W.Y. Ye, J.Q. Wang, S.J. Wang, P. Ji, G.Y. Zhou, G.P. Zhao, H.L. Ge, Y. Wang, dCTP pyrophosphohydrase exhibits nucleic accumulation in multiple carcinomas , European Journal of Histochemistry: Vol. 57 No. 3 (2013)
- Xuanjin Zhu, Weilu Jia, Yong Yan, Yong Huang, Bailin Wang, NOP14 regulates the growth, migration, and invasion of colorectal cancer cells by modulating the NRIP1/GSK-3β/β-catenin signaling pathway , European Journal of Histochemistry: Vol. 65 No. 3 (2021)
- Huiwen Li, Bo Huang, miR-19a targeting CLCA4 to regulate the proliferation, migration, and invasion of colorectal cancer cells , European Journal of Histochemistry: Vol. 66 No. 1 (2022)
- Jindong Li, Jie Kang, Weiyan Liu, Jiazhe Liu, Gaofeng Pan, Anwei Mao, Qing Zhang, Jingfeng Lu, Junbin Ding, Hongchang Li, Docetaxel-resistant triple-negative breast cancer cell-derived exosomal lncRNA LINC00667 reduces the chemosensitivity of breast cancer cells to docetaxel via targeting miR-200b-3p/Bcl-2 axis , European Journal of Histochemistry: Vol. 66 No. 4 (2022)
- Azzurra Margiotta, Cinzia Progida, Oddmund Bakke, Cecilia Bucci, Characterization of the role of RILP in cell migration , European Journal of Histochemistry: Vol. 61 No. 2 (2017)
- Ying Wang, Shifa Yuan, Jing Ma, Hong Liu, Lizhen Huang, Fengzhen Zhang, Substance P is overexpressed in cervical squamous cell carcinoma and promoted proliferation and invasion of cervical cancer cells in vitro , European Journal of Histochemistry: Vol. 67 No. 3 (2023)
You may also start an advanced similarity search for this article.
Publication Facts
Metric
This article
Other articles
Peer reviewers
2
2.4
Reviewer profiles N/A
Author statements
Author statements
This article
Other articles
Data availability
N/A
16%
External funding
N/A
32%
Competing interests
N/A
11%
Metric
This journal
Other journals
Articles accepted
57%
33%
Days to publication
71
145
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy

 https://doi.org/10.4081/ejh.2021.3214
https://doi.org/10.4081/ejh.2021.3214











