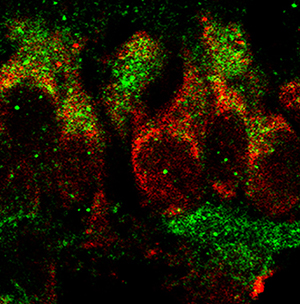
Secreted key regulators (Fgf1, Bmp4, Gdf3) are expressed by PAC1-immunopositive retinal ganglion cells in the postnatal rat retina
Submitted: 17 December 2021
Accepted: 2 March 2022
Published: 27 April 2022
Accepted: 2 March 2022
Abstract Views: 950
PDF: 553
HTML: 19
HTML: 19
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Yuka Maruyama-Koide, Sumiko Mikawa, Takeshi Sasaki, Kohji Sato, Bone morphogenetic protein-4 and bone morphogenetic protein receptors expressions in the adult rat eye , European Journal of Histochemistry: Vol. 61 No. 3 (2017)
- W. Romero-Fernandez, D.O. Borroto-Escuela, V. Vargas-Barroso, M. Narváez, M. Di Palma, L.F. Agnati, J. Larriva Sahd, K. Fuxe, Dopamine D1 and D2 receptor immunoreactivities in the arcuate-median eminence complex and their link to the tubero-infundibular dopamine neurons , European Journal of Histochemistry: Vol. 58 No. 3 (2014)
- A. Makowiecka, A. Simiczyjew, D. Nowak, A.J. Mazur, Varying effects of EGF, HGF and TGFβ on formation of invadopodia and invasiveness of melanoma cell lines of different origin , European Journal of Histochemistry: Vol. 60 No. 4 (2016)
- Azzurra Margiotta, Cinzia Progida, Oddmund Bakke, Cecilia Bucci, Characterization of the role of RILP in cell migration , European Journal of Histochemistry: Vol. 61 No. 2 (2017)
- S.A. Ferreira, J.L.A. Vasconcelos, R.C.W.C. Silva, C.L.B. Cavalcanti, C.L. Bezerra, M.J.B.M. Rêgo, E.I.C. Beltrão, Expression patterns of α2,3-Sialyltransferase I and α2,6-Sialyltransferase I in human cutaneous epithelial lesions , European Journal of Histochemistry: Vol. 57 No. 1 (2013)
- X.C. Xu, X. Abuduhadeer, W.B. Zhang, T. Li, H. Gao, Y.H. Wang, Knockdown of RAGE inhibits growth and invasion of gastric cancer cells , European Journal of Histochemistry: Vol. 57 No. 4 (2013)
- Xilin Ge, Caoxin Huang, Wenting Chen, Chen Yang, Wenfang Huang, Jia Li, Shuyu Yang, Effect of Danggui Buxue decoction on hypoxia-induced injury of retinal Müller cells in vitro , European Journal of Histochemistry: Vol. 68 No. 4 (2024)
- G. Scarsella, M. Nebbioso, S. Stefanini, N. Pescosolido, Degenerative effects in rat eyes after experimental ocular hypertension , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- D. Nowak, A. J. Mazur, A. Popow-Woźniak, A. Radwańska, H. G. Mannherz, M. Malicka-Błaszkiewicz, Subcellular distribution and expression of cofilin and ezrin in human colon adenocarcinoma cell lines with different metastatic potential , European Journal of Histochemistry: Vol. 54 No. 2 (2010)
- J.P. Damico, E. Ervolino, K.R. Torres, D.S. Batagello, R.J. Cruz-Rizzolo, C.A. Casatti, J.A. Bauer, Phenotypic alterations of neuropeptide Y and calcitonin gene-related peptide-containing neurons innervating the rat temporomandibular joint during carrageenan-induced arthritis , European Journal of Histochemistry: Vol. 56 No. 3 (2012)
You may also start an advanced similarity search for this article.
Publication Facts
Metric
This article
Other articles
Peer reviewers
2
2.4
Reviewer profiles N/A
Author statements
Author statements
This article
Other articles
Data availability
N/A
16%
External funding
N/A
32%
Competing interests
N/A
11%
Metric
This journal
Other journals
Articles accepted
57%
33%
Days to publication
130
145
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy

 https://doi.org/10.4081/ejh.2022.3373
https://doi.org/10.4081/ejh.2022.3373












