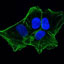
PRMT1 arginine methyltransferase accumulates in cytoplasmic bodies that respond to selective inhibition and DNA damage
Submitted: 25 February 2014
Accepted: 17 March 2014
Published: 2 May 2014
Accepted: 17 March 2014
Abstract Views: 2470
PDF: 601
Supplementary: 283
HTML: 793
Supplementary: 283
HTML: 793
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Martha I. Dávila-Rodríguez, Elva I. Cortés-Gutiérrez, Roberto Hernández-Valdés, Karla Guzmán-Cortés, Rosa E. De León-Cantú, Ricardo M. Cerda-Flores, Enrique Báez-De la Fuente, DNA damage in acute myeloid leukemia patients of Northern Mexico , European Journal of Histochemistry: Vol. 61 No. 4 (2017)
- Claudio Casali, Stella Siciliani, Lorena Zannino, Marco Biggiogera, Histochemistry for nucleic acid research: 60 years in the European Journal of Histochemistry , European Journal of Histochemistry: Vol. 66 No. 2 (2022)
- E. Coluccia, G. Pichiri, M. Nieddu, P. Coni, S. Manconi, A.M. Deiana, S. Salvadori, R. Mezzanotte, Identification of two new repetitive elements and chromosomal mapping of repetitive DNA sequences in the fish Gymnothorax unicolor (Anguilliformes: Muraenidae) , European Journal of Histochemistry: Vol. 55 No. 2 (2011)
- Hui Xu, Yanbo Chen, Qi Chen, Huan Xu, Yanxia Wang, Jiao Yu, Juan Zhou, Zhong Wang, Bing Xu, DNMT1 regulates IL-6- and TGF-β1-induced epithelial mesenchymal transition in prostate epithelial cells , European Journal of Histochemistry: Vol. 61 No. 2 (2017)
- E. Ayarza, M. González, F. López, R. Fernández-Donoso, J. Page, S. Berrios, Alterations in chromosomal synapses and DNA repair in apoptotic spermatocytes of Mus m. domesticus , European Journal of Histochemistry: Vol. 60 No. 2 (2016)
- Elva I. Cortés Gutiérrez, Catalina García-Vielma, Adriana Aguilar-Lemarroy, Veronica Vallejo-Ruíz, Patricia Piña-Sánchez, Pablo Zapata-Benavides, Jaime Gosalvez, Expression of the HPV18/E6 oncoprotein induces DNA damage , European Journal of Histochemistry: Vol. 61 No. 2 (2017)
- E. I. Cortés-Gutiérrez, M. I. Davila-RodrÃguez, J. L. Fernández, C. López-Fernández, J. Gosalvez, DNA breakage detection-fluorescence in situ hybridization (DBD-FISH) in buccal cells , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- G. Lattanzi, S. Marmiroli, A. Facchini, N.M. Maraldi, Nuclear damages and oxidative stress: new perspectives for laminopathies , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- Francesca Diomede, Soundara Rajan Thangavelu, Ilaria Merciaro, Monica D'Orazio, Placido Bramanti, Emanuela Mazzon, Oriana Trubiani, Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: role of epigenetic modifications to the inflammation , European Journal of Histochemistry: Vol. 61 No. 3 (2017)
- E. I. Cortés-Gutiérrez, M. I. Dávila-RodrÃguez, J. L. Fernández, C. López-Fernández, J. Gosálvez, Koilocytes are enriched for alkaline-labile sites , European Journal of Histochemistry: Vol. 54 No. 4 (2010)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2014.2389
https://doi.org/10.4081/ejh.2014.2389