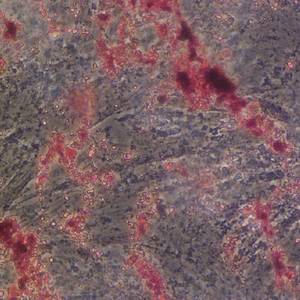
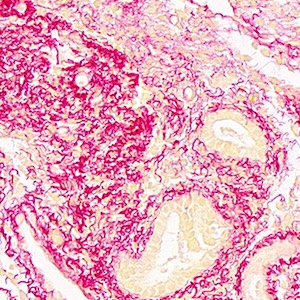
Overexpression of kynurenic acid and 3-hydroxyanthranilic acid after rat traumatic brain injury
Submitted: 3 October 2018
Accepted: 2 November 2018
Published: 14 November 2018
Accepted: 2 November 2018
Abstract Views: 1531
PDF: 635
HTML: 16
HTML: 16
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Arturo Mangas, Javier Yajeya, Noelia González, Isabel Ruiz, Marianny Pernía, Michel Geffard, Rafael Coveñas, Gemst: a taylor-made combination that reverts neuroanatomical changes in stroke , European Journal of Histochemistry: Vol. 61 No. 2 (2017)
- E. Akat, H. Arıkan, B. Göçmen, Histochemical and biometric study of the gastrointestinal system of Hyla orientalis (Bedriaga, 1890) (Anura, Hylidae) , European Journal of Histochemistry: Vol. 58 No. 4 (2014)
- Yankun Wang, Yuning Liu, Yawei Wang, Ao Zhang, Wenqian Xie, Haolin Zhang, Qiang Weng, Meiyu Xu, Investigation of seasonal changes in lipid synthesis and metabolism-related genes in the oviduct of Chinese brown frog (Rana dybowskii) , European Journal of Histochemistry: Vol. 67 No. 4 (2023)
- M. Lehmann, F. Martin, K. Mannigel, K. Kaltschmidt, U. Sack, U. Anderer, Three-dimensional scaffold-free fusion culture: the way to enhance chondrogenesis of in vitro propagated human articular chondrocytes , European Journal of Histochemistry: Vol. 57 No. 4 (2013)
- A. Mangas, J. Yajeya, N. González, I. Ruiz, M. Geffard, R. Coveñas, 3-hydroxi-anthranilic acid is early expressed in stroke , European Journal of Histochemistry: Vol. 60 No. 4 (2016)
- M. J. Galvão, A. Santos, M. D. Ribeiro, A. Ferreira, F. Nolasco, Optimization of the tartrate-resistant acid phosphatase detection by histochemical method , European Journal of Histochemistry: Vol. 55 No. 1 (2011)
- D. Ami, M. Di Segni, M. Forcella, V. Meraviglia, M. Baccarin, S.M. Doglia, G. Terzoli, Role of water in chromosome spreading and swelling induced by acetic acid treatment: a FTIR spectroscopy study , European Journal of Histochemistry: Vol. 58 No. 1 (2014)
- L. Vinci, A. Ravarino, V. Fanos, A.G. Naccarato, G. Senes, C. Gerosa, G. Bevilacqua, G. Faa, R. Ambu, Immunohistochemical markers of neural progenitor cells in the early embryonic human cerebral cortex , European Journal of Histochemistry: Vol. 60 No. 1 (2016)
- S. Strobel, J.A. Encarnação, N.I. Becker, T.E. Trenczek, Histological and histochemical analysis of the gastrointestinal tract of the common pipistrelle bat (Pipistrellus pipistrellus) , European Journal of Histochemistry: Vol. 59 No. 2 (2015)
- I.M.S. Paulsen, H. Dimke, S. Frische, A single simple procedure for dewaxing, hydration and heat-induced epitope retrieval (HIER) for immunohistochemistry in formalin fixed paraffin-embedded tissue , European Journal of Histochemistry: Vol. 59 No. 4 (2015)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2018.2985
https://doi.org/10.4081/ejh.2018.2985