Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Expressions of ZNF436, β-catenin, EGFR, and CMTM5 in breast cancer and their clinical significances
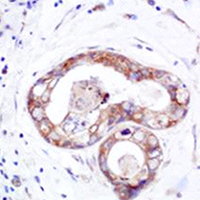
As the leading malignancy among women, breast cancer is a serious threat to the life and health of women. In this context, it is of particular importance that a proper therapeutic target be identified for breast cancer treatment. We collected the pathological tissues of 80 patients, with the view to discovering appropriate molecular targets for the treatment of breast cancer, this paper analyzes the expressions of ZNF436, β-catenin, EGFR and CMTM5 in breast cancer tissues, as well as their correlations with breast cancer in combination with the clinicopathologic characteristics of studied patients. Immunohistochemistry (IHC) was utilized to detect the expression levels of ZNF436, β-catenin, EGFR and CMTM5 in cancerous and paracancerous tissues of breast cancer patients. The expression levels of ZNF436, β-Catenin and EGFR in breast cancer tissues were significantly greater than those in paracancerous tissues in this study (p<0.05), while CMTM5 was highly expressed in paracancerous tissues (p<0.05). Additionally, the correlation of the expressions of such indicators with the staging, differentiation and lymphatic metastasis of breast cancer, were also found to be statistically significant at the level p<0.05. The different expression levels of ZNF436, β-catenin, EGFR and CMTM5 in breast cancer and paracancerous tissues open up the possibility of utilizing them as molecular markers for breast cancer. These findings provide a theoretical basis for targeted molecular therapies for breast cancer, and hence carry a significant practical significance.
Altmetrics
Downloads
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.