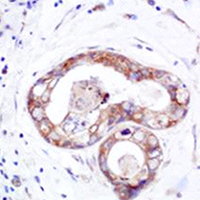
Expressions of ZNF436, β-catenin, EGFR, and CMTM5 in breast cancer and their clinical significances
Submitted: 13 August 2020
Accepted: 7 December 2020
Published: 20 January 2021
Accepted: 7 December 2020
Abstract Views: 1459
PDF: 596
Supplementary: 131
HTML: 11
Supplementary: 131
HTML: 11
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- A. Arcucci, M.R. Ruocco, F. Albano, G. Granato, V. Romano, G. Corso, C. Bancone, E. De Vendittis, A. Della Corte, S. Montagnani, Analysis of extracellular superoxide dismutase and Akt in ascending aortic aneurysm with tricuspid or bicuspid aortic valve , European Journal of Histochemistry: Vol. 58 No. 3 (2014)
- Mihály Kálmán, Erzsébet Oszwald, István Adorján, Appearance of β-dystroglycan precedes the formation of glio-vascular end-feet in developing rat brain , European Journal of Histochemistry: Vol. 62 No. 2 (2018)
- Cecilia Dall'Aglio, Francesca Mercati, Valerio Faeti, Gabriele Acuti, Massimo Trabalza Marinucci, Elena De Felice, Federico Maria Tardella, Maria Pia Franciosini, Patrizia Casagrande Proietti, Daniele Catorci, Paolo Stacchini, Augusto Pastorelli, Paola Scocco, Immuno- and glyco-histochemistry as a tool to evaluate the oregano supplemented feed effects in pig gut , European Journal of Histochemistry: Vol. 64 No. 1 (2020)
- Qingjing Gao, Wenqian Xie, Wenjing Lu, Yuning Liu, Haolin Zhang, Yingying Han, Qiang Weng, Seasonal patterns of prolactin, prolactin receptor, and STAT5 expression in the ovaries of wild ground squirrels (Citellus dauricus Brandt) , European Journal of Histochemistry: Vol. 67 No. 4 (2023)
- Jinshuang Li, Dawei Xu, Ce Shi, Chunqi Cheng, Ziheng Xu, Xingjuan Gao, Yong Cheng, Alarin regulates RyR2 and SERCA2 to improve cardiac function in heart failure with preserved ejection fraction , European Journal of Histochemistry: Vol. 68 No. 4 (2024)
- B Vitolo, MR Lidonnici, C Montecucco, A Montecucco, A new monoclonal antibody against DNA ligase I is a suitable marker of cell proliferation in cultured cell and tissue section samples , European Journal of Histochemistry: Vol. 49 No. 4 (2005)
- L. Fassina, G. Magenes, A. Inzaghi, S. Palumbo, G. Allavena, C. Miracco, L. Pirtoli, M. Biggiogera, S. Comincini, AUTOCOUNTER, an ImageJ JavaScript to analyze LC3B-GFP expression dynamics in autophagy-induced astrocytoma cells , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- M. Monti, In vivo cellular imaging using fluorescent proteins - Methods and Protocols , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- Gianpaolo Antonio Basile, Salvatore Bertino, Alessia Bramanti, Rosella Ciurleo, Giuseppe Pio Anastasi, Demetrio Milardi, Alberto Cacciola, Striatal topographical organization: Bridging the gap between molecules, connectivity and behavior , European Journal of Histochemistry: Vol. 65 No. s1 (2021): Special Collection on Advances in Neuromorphology in Health and Disease
- Dajie Wang, Zhaofeng Zhou, Liang Yuan, Polydatin reverses oxidation low lipoprotein (oxLDL)-induced apoptosis of human umbilical vein endothelial cells via regulating the miR-26a-5p/BID axis , European Journal of Histochemistry: Vol. 66 No. 4 (2022)
<< < 35 36 37 38 39 40 41 42 43 44 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2021.3173
https://doi.org/10.4081/ejh.2021.3173










