Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Immunopositivity for Siglec-15 in gastric cancer and its association with clinical and pathological parameters
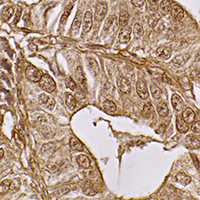
The sialic acid-binding immunoglobulin-type lectin Siglec-15 is a promising target to cancer immunotherapy in several tumor types. The present study aimed to investigate Siglec-15 expression in gastric cancer (GC) patient tissue and to evaluate its clinical value. Siglec-15 expression was evaluated by immunohistochemistry with 71 patients. Siglec-15 staining was observed in tumor cells of 53 (74.64%) patients, with significant association with histologic classification and angiolymphatic invasion (p<0.05). Immunohistochemistry analysis also detected Siglec-15 in tumor-associated stroma cells (macrophages/myeloid cells). There was no significant association with outcomes parameters. Siglec-15 expression in well differentiated histological GC tissues and in the tumor microenvironment are potential targets to be further investigated as a novel prognostic factor for GC.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
Citations
10.3389/fonc.2022.1073932
10.1007/s12020-023-03452-1
10.1002/cjp2.260
10.1124/molpharm.121.000470
10.1186/s12885-024-12061-8
10.3389/fonc.2024.1437006
10.3390/ijms26031156
10.3389/fimmu.2022.975787
10.1002/cjp2.303
10.3389/fsurg.2022.898733
10.3389/fimmu.2024.1490505
10.3390/diagnostics11122370
10.1016/j.semcancer.2025.01.007
10.1186/s13045-024-01638-2
10.1080/2162402X.2024.2376264
10.1016/j.heliyon.2024.e25266
10.1177/02841851241286109
10.3389/fimmu.2021.790317
10.1111/crj.13772
Ethics Approval
Ethical approval was obtained from the Human Ethics Committee of the Hospital do Câncer de Pernambuco (HCP) (CAAE: 39976214.90000.5205).Supporting Agencies
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3174
https://doi.org/10.4081/ejh.2021.3174