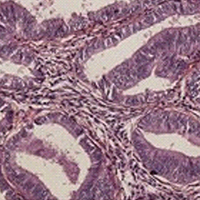
PD-L1 expression with QR1 and E1L3N antibodies according to histological ovarian cancer subtype: A series of 232 cases
Submitted: 1 October 2020
Accepted: 10 February 2021
Published: 10 March 2021
Accepted: 10 February 2021
Abstract Views: 970
PDF: 513
HTML: 14
HTML: 14
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- C Sarasquete, M Gutiérrez, New Tetrachromic VOF Stain (Type III-G.S) for Normal and Pathological Fish Tissues , European Journal of Histochemistry: Vol. 49 No. 2 (2005)
- B Bilinska, M Kotula-Balak, J Sadowska, Morphology and function of human Leydig cells in vitro. Immunocytochemical and radioimmunological analyses , European Journal of Histochemistry: Vol. 53 No. 1 (2009)
- A. Bolekova, D. Kluchova, L. Tomasova, N. Hvizdosova, Effect of retinoic acid on the nitrergic innervation of meibomian glands in rats , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
You may also start an advanced similarity search for this article.
Publication Facts
Metric
This article
Other articles
Peer reviewers
2
2.4
Reviewer profiles N/A
Author statements
Author statements
This article
Other articles
Data availability
N/A
16%
External funding
N/A
32%
Competing interests
N/A
11%
Metric
This journal
Other journals
Articles accepted
57%
33%
Days to publication
159
145
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy

 https://doi.org/10.4081/ejh.2021.3185
https://doi.org/10.4081/ejh.2021.3185











