Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Effects of rutin on osteoblast MC3T3-E1 differentiation, ALP activity and Runx2 protein expression
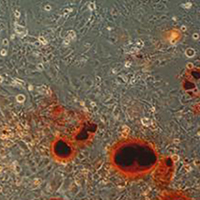
As a flavonoid, rutin has been found to have a wide range of biological functions, such as resisting inflammation and oxidation, and preventing cerebral hemorrhage and hypertension. It has been found to play an important role in osteoporosis and other orthopedic diseases in recent years. MC3T3-E1 cells were randomly divided into a control group, a rutin-1 group (0.01 mmol/L), a rutin-2 group (0.05 mmol/L) and a rutin-3 group (0.1 mmol/L). Osteogenic differentiation of cells was induced by osteogenic induction fluid. The control group was treated with the maximum dose of drug solvent. 2~3 days later, the solvent was replaced with fresh osteogenic induction fluid containing rutin. After a certain period of routine culture, the cells were collected for subsequent experiments. The expression of Runx2 gene in cells in all groups was detected by Real-time PCR; the expression of Runx2 protein was detected by Western blot and immunocytochemistry; the activity of ALP was detected by reagent kit method; osteogenic differentiation was analyzed by alizarin red staining. The results of Real-time PCR showed that, compared with the control group, the treatment of cells with rutin can significantly increase the expression of Runx2 gene (p<0.05); the higher the concentration, the higher the expression of Runx2 gene, and significant differences were found among groups in which different concentrations were used (p<0.05); the results of Western blot and IHC showed that the expression trend of Runx2 protein in each group was consistent with PCR results. In drug treatment groups, the activity of ALP was significantly higher than that in the control group (p<0.05); there were significant differences among groups in which different concentrations were used (p<0.05). The results of alizarin red staining showed that calcified nodules were formed in all groups and that the area of calcified nodules formed in groups treated with rutin was greater than that in the control group; the greater the concentration, the larger the area. Rutin can promote osteoblastic differentiation; and the greater the concentration, the more effective it is.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.