Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Immunoreactivities of AR, ERα, ERβ and aromatase in the nuptial pad of Chinese brown frog (Rana dybowskii) during pre-hibernation and the breeding period
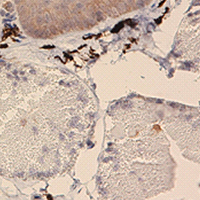
There is a prominent local raised pad called nuptial pad on the forelimb of Chinese brown frog (Rana dybowskii), which is hypothetically concluded as an enhancement of the grip and a spreader of pheromone during the amplexus. In this study, we investigated the immunolocalization and protein expression levels of AR, ERα, ERβ and aromatase in the nuptial pad of R. dybowskii during pre-hibernation and the breeding period. Histologically, the annual development of the nuptial pad in R. dybowskii is manifested as the larger area of specialized mucous gland and the longer length of papillary epidermal projection during the breeding period. AR, ERα, ERβ and aromatase are present in the stratum granulosum, stratum spinosum, stratum basale and the secretory portion of specialized mucous glands during both periods. Western blotting results confirmed that AR, ERα and ERβ protein levels are higher during pre-hibernation than those during the breeding season. These results suggest that nuptial pad is the direct target organ of androgen and estrogen. Androgen may participate in the regulation of annual development and glandular function of nuptial pad, and estrogen may play an endocrine, autocrine or paracrine role during pre-hibernation and the breeding period.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3206
https://doi.org/10.4081/ejh.2021.3206