Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Seasonal expressions of VEGF and its receptors VEGFR1 and VEGFR2 in the prostate of the wild ground squirrels (Spermophilus dauricus)
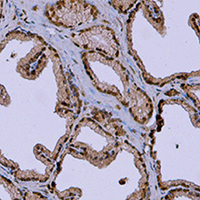
As a vital male accessory reproductive gonad, the prostate requires vascular endothelial growth factors for promoting its growth and development. In this study, we investigated the localizations and expressions of vascular endothelial growth factor (VEGF) and its receptors including VEGF-receptor1 (VEFGR1) and VEGF-receptor2 (VEGFR2) in the prostate of the wild ground squirrels during the breeding and the non-breeding seasons. The values of total prostate weight and volume in the breeding season were higher than those in the non-breeding season. Histological observations showed that the exocrine lumens of the prostate expanded in the breeding season and contracted in the non-breeding season. The mRNA expression levels of VEGF and VEGFR2 in the prostate were higher in the breeding season than those in the non-breeding season, but the mRNA expression level of VEGFR1 had no significant change between the breeding and non-breeding seasons. Immunohistochemical results revealed that VEGF, VEGFR1 and VEGFR2 were presented in epithelial and stromal cells during the breeding and non-breeding seasons. In addition, the microvessels of the prostate were widely distributed and the number of microvessels increased obviously in the breeding season, while decreased sharply in the non-breeding season. These results suggested that expression levels of VEGF and VEGFR2 might be correlated with seasonal changes in morphology and functions of the prostate, and VEGF might serve as pivotal regulators to affect seasonal changes in the prostate functions of the wild male ground squirrels via an autocrine/paracrine pathway.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
Ethics Approval
All the procedures on animals were carried out in accordance with the policy on the Care and Use of Animals by the Ethical Committee, Beijing Forestry University and approved by the Department of Agriculture of Hebei province, China (JNZF11/2007).Supporting Agencies
National Natural Science Foundation of China, Natural Science Foundation of Beijing, Young Scientist Start-up funding of Beijing Forestry UniversityHow to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3219
https://doi.org/10.4081/ejh.2021.3219