Quantitative, structural and molecular changes in neuroglia of aging mammals: A review
Accepted: 27 May 2021
HTML: 11
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
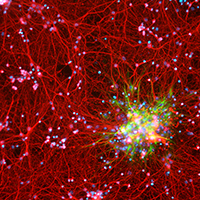
The neuroglia of the central and peripheral nervous systems undergo numerous changes during normal aging. Astrocytes become hypertrophic and accumulate intermediate filaments. Oligodendrocytes and Schwann cells undergo alterations that are often accompanied by degenerative changes to the myelin sheath. In microglia, proliferation in response to injury, motility of cell processes, ability to migrate to sites of neural injury, and phagocytic and autophagic capabilities are reduced. In sensory ganglia, the number and extent of gaps between perineuronal satellite cells – that leave the surfaces of sensory ganglion neurons directly exposed to basal lamina– increase significantly. The molecular profiles of neuroglia also change in old age, which, in view of the interactions between neurons and neuroglia, have negative consequences for important physiological processes in the nervous system. Since neuroglia actively participate in numerous nervous system processes, it is likely that not only neurons but also neuroglia will prove to be useful targets for interventions to prevent, reverse or slow the behavioral changes and cognitive decline that often accompany senescence.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.
Similar Articles
- T. Petr, V. Å mÃd, J. Å mÃdová, H. Hůlková, M. Jirkovská, M. Elleder, L. Muchová, L. Vitek, F. Å mÃd, Histochemical detection of GM1 ganglioside using cholera toxin-B subunit. Evaluation of critical factors optimal for in situ detection with special emphasis to acetone pre-extraction , European Journal of Histochemistry: Vol. 54 No. 2 (2010)
- MA Croce, F Boraldi, D Quaglino, R Tiozzo, I Pasquali-Ronchetti, Hyaluronan uptake by adult human skin fibroblasts in vitro , European Journal of Histochemistry: Vol. 47 No. 1 (2003)
- A Kora, M Vere, V Davidovi, Insulin-induced iron loading in the rat brown adipose tissue: histochemical and electron-microscopic study , European Journal of Histochemistry: Vol. 47 No. 3 (2003)
- CJ Woodall, DI Graham, Evidence for neuronal localisation of enteroviral sequences in motor neurone disease/amyotrophic lateral sclerosis by in situ hybridization , European Journal of Histochemistry: Vol. 48 No. 2 (2004)
- Cecilia Dall'Aglio, Paola Scocco, Margherita Maranesi, Linda Petrucci, Gabriele Acuti, Elena De Felice, Francesca Mercati, Immunohistochemical identification of resistin in the uterus of ewes subjected to different diets: Preliminary results , European Journal of Histochemistry: Vol. 63 No. 2 (2019)
- E Wyroba, L Surmacz, M Osinska, J Wiejak, Phagosome maturation in unicellular eukaryote Paramecium: the presence of RILP, Rab7 and LAMP-2 homologues , European Journal of Histochemistry: Vol. 51 No. 3 (2007)
- M Malatesta, F Perdoni, S Muller, C Zancanaro, C Pellicciari, Nuclei of aged myofibres undergo structural and functional changes suggesting impairment in RNA processing , European Journal of Histochemistry: Vol. 53 No. 2 (2009)
- A Pugnaloni, GL Sgarbi, M Tesei, M D'Aurelio, G Ragni, G Parenti Castelli, S Salardi, S Zucchini, C Bovina, E Cacciari, G Lenaz, G Biagini, Lymphocyte dysmetabolism: an immunocytochemical comparative approach in IDDM and control subjects , European Journal of Histochemistry: Vol. 45 No. 1 (2001)
- M. Onisto, S. Garbisa, M. Spina, Lorenzo Gotte (1926-1991): a pioneer of elastin , European Journal of Histochemistry: Vol. 60 No. 3 (2016)
- A Gigante, C Bevilacqua, A Pagnotta, S Manzotti, A Toesca, F Greco, Expression of NGF, Trka and p75 in human cartilage , European Journal of Histochemistry: Vol. 47 No. 4 (2003)
<< < 79 80 81 82 83 84 85 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2021.3249
https://doi.org/10.4081/ejh.2021.3249










