Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
From the intestinal mucosal barrier to the enteric neuromuscular compartment: an integrated overview on the morphological changes in Parkinson’s disease
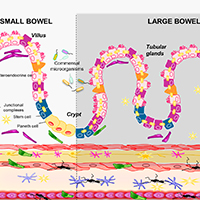
Gastrointestinal dysfunctions represent the most common non-motor symptoms in Parkinson’s disease (PD). Of note, changes in gut microbiota, impairments of intestinal epithelial barrier (IEB), bowel inflammation and neuroplastic rearrangements of the enteric nervous system (ENS) could be involved in the pathophysiology of the intestinal disturbances in PD. In this context, although several review articles have pooled together evidence on the alterations of enteric bacteria-neuro-immune network in PD, a revision of the literature on the specific morphological changes occurring in the intestinal mucosal barrier, the ENS and enteric muscular layers in PD, is lacking. The present review provides a complete appraisal of the available knowledge on the morphological alterations of intestinal mucosal barrier, with particular focus on IEB, ENS and enteric muscular layers in PD. In particular, our intent was to critically discuss whether, based on evidence from translational studies and pre-clinical models, morphological changes in the intestinal barrier and enteric neuromuscular compartment contribute to the pathophysiology of intestinal dysfunctions occurring in PD.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
Citations
10.1016/S2468-1253(22)00241-2
10.1177/10738584231163460
10.1016/j.smim.2023.101846
10.1016/j.phrs.2023.106787
10.1016/j.arr.2022.101812
10.1016/j.labinv.2023.100194
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3278
https://doi.org/10.4081/ejh.2021.3278