Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Changes in cytoplasmic and extracellular neuromelanin in human substantia nigra with normal aging
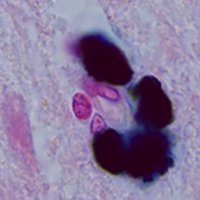
Neuromelanin (NM) is a dark polymer pigment produced in certain populations of catecholaminergic neurons in the brain. It is present in various areas of the human brain, most often in the substantia nigra (SN) pars compacta and the locus coeruleus, the main centers of dopaminergic and noradrenergic innervation, respectively. Interest in NM has revived in recent years due to the alleged link between NM and the particular vulnerability of neuromelanin-containing neurons to neurodegeneration. The aim of this work was to study the structural, cytochemical, and localization features of cytoplasmic and extracellular neuromelanin in the human SN pars compacta during normal aging. Sections of human SN from young/middle-aged adults (25 to 51 years old, n=7) and older adults (60 to 78 years old, n=5), all of which had no neurological disorders, were stained histochemically for metals (Perls’ reaction, Mayer's hematoxylin) and immunohistochemically for tyrosine hydroxylase (TH) and Iba-1. It was shown that dopaminergic neurons in SN pars compacta differ in the amount of neuromelanin and the intensity of TH-immunoreactivity. The number of neuromelanin-containing neurons with decreased TH-immunoreactivity positively correlates with age. Extracellular NM is present in SN pars compacta in both young/middle-aged and older adults. The number of extracellular NM accumulations increases with aging. Cytoplasmic and extracellular NM are predominantly not stained using histochemical methods for detecting metals in people of all ages. We did not detect the appearance of amoeboid microglia in human SN pars compacta with aging, but we found an age-related increase in microglial phagocytic activity. The absence of pronounced microgliosis, as well as a pronounced loss of neuromelanin-containing neurons, indicate the absence of neuroinflammation in human SN pars compacta during normal aging.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2021.3283
https://doi.org/10.4081/ejh.2021.3283