Striatal topographical organization: Bridging the gap between molecules, connectivity and behavior
Accepted: 7 September 2021
HTML: 16
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
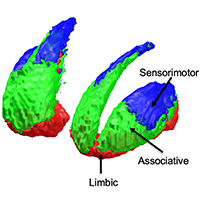
The striatum represents the major hub of the basal ganglia, receiving projections from the entire cerebral cortex and it is assumed to play a key role in a wide array of complex behavioral tasks. Despite being extensively investigated during the last decades, the topographical organization of the striatum is not well understood yet. Ongoing efforts in neuroscience are focused on analyzing striatal anatomy at different spatial scales, to understand how structure relates to function and how derangements of this organization are involved in various neuropsychiatric diseases. While being subdivided at the macroscale level into dorsal and ventral divisions, at a mesoscale level the striatum represents an anatomical continuum sharing the same cellular makeup. At the same time, it is now increasingly ascertained that different striatal compartments show subtle histochemical differences, and their neurons exhibit peculiar patterns of gene expression, supporting functional diversity across the whole basal ganglia circuitry. Such diversity is further supported by afferent connections which are heterogenous both anatomically, as they originate from distributed cortical areas and subcortical structures, and biochemically, as they involve a variety of neurotransmitters. Specifically, the cortico-striatal projection system is topographically organized delineating a functional organization which is maintained throughout the basal ganglia, subserving motor, cognitive and affective behavioral functions. While such functional heterogeneity has been firstly conceptualized as a tripartite organization, with sharply defined limbic, associative and sensorimotor territories within the striatum, it has been proposed that such territories are more likely to fade into one another, delineating a gradient-like organization along medio-lateral and ventro-dorsal axes. However, the molecular and cellular underpinnings of such organization are less understood, and their relations to behavior remains an open question, especially in humans. In this review we aimed at summarizing the available knowledge on striatal organization, especially focusing on how it links structure to function and its alterations in neuropsychiatric diseases. We examined studies conducted on different species, covering a wide array of different methodologies: from tract-tracing and immunohistochemistry to neuroimaging and transcriptomic experiments, aimed at bridging the gap between macroscopic and molecular levels.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.
Similar Articles
- K Zuwala, F Merigo, C Zancanaro, Neuronal intermediate filaments in the developing tongue of the frog Rana esculenta , European Journal of Histochemistry: Vol. 48 No. 2 (2004)
- L. Fassina, G. Magenes, A. Inzaghi, S. Palumbo, G. Allavena, C. Miracco, L. Pirtoli, M. Biggiogera, S. Comincini, AUTOCOUNTER, an ImageJ JavaScript to analyze LC3B-GFP expression dynamics in autophagy-induced astrocytoma cells , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- H. Xu, X. Zhang, H. Wang, Y. Zhang, Y. Shi, X. Zhang, Continuous cyclic mechanical tension increases ank expression in endplate chondrocytes through the TGF-β1 and p38 pathway , European Journal of Histochemistry: Vol. 57 No. 3 (2013)
- Zhen Wang, Xiaoyan Du, Daoyang Yu, Yang Yang, Gaoen Ma, Xueli Jia, Lulu Cheng, Sufentanil alleviates cerebral ischemia-reperfusion injury by inhibiting inflammation and protecting the blood-brain barrier in rats , European Journal of Histochemistry: Vol. 66 No. 1 (2022)
- Yin Pan, Di Qiu, Shu Chen, Xiaoxue Han, Ruiman Li, High glucose inhibits neural differentiation by excessive autophagy via peroxisome proliferator-activated receptor gamma , European Journal of Histochemistry: Vol. 67 No. 2 (2023)
- Fenqiang Qi, Yuxin Deng, Wei Huang, Yanli Cai, Kelin Hong, Shui Xiang, Irisin suppresses PDGF-BB-induced proliferation of vascular smooth muscle cells in vitro by activating AMPK/mTOR-mediated autophagy , European Journal of Histochemistry: Vol. 68 No. 4 (2024)
- S Bettini, F Ciani, V Franceschini, Cell proliferation and growth-associated protein 43 expression in the olfactory epithelium in Poecilia reticulata after copper solution exposure , European Journal of Histochemistry: Vol. 50 No. 2 (2006)
- T. Lorenzi, A. Turi, C. Crescimanno, M. Morroni, M. Castellucci, G. David, A. L. Tranquilli, D. Marzioni, Syndecan expressions in the human amnion and chorionic plate , European Journal of Histochemistry: Vol. 54 No. 4 (2010)
- G. Lattanzi, S. Marmiroli, A. Facchini, N.M. Maraldi, Nuclear damages and oxidative stress: new perspectives for laminopathies , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- Ewing Duque-Díaz, Hernán Hurtado Giraldo, Linda P. Rocha-Muñoz, Rafael Coveñas, Glyphosate, AMPA and glyphosate-based herbicide exposure leads to GFAP, PCNA and caspase-3 increased immunoreactive area on male offspring rat hypothalamus , European Journal of Histochemistry: Vol. 66 No. 4 (2022)
<< < 12 13 14 15 16 17 18 19 20 21 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2021.3284
https://doi.org/10.4081/ejh.2021.3284










