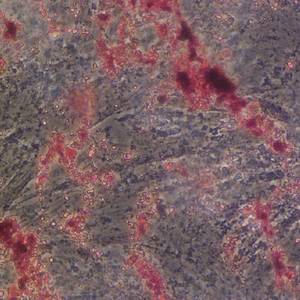
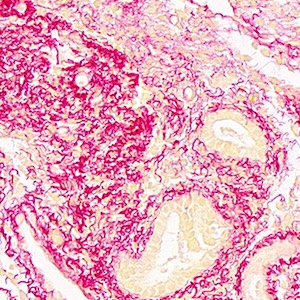
Seasonal expressions of GPR41 and GPR43 in the colon of the wild ground squirrels (Spermophilus dauricus)
Submitted: 23 October 2021
Accepted: 2 January 2022
Published: 21 January 2022
Accepted: 2 January 2022
Abstract Views: 1246
PDF: 590
HTML: 27
HTML: 27
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- H. Zhang, Y. Wang, J. Zhang, L. Wang, Q. Li, X. Sheng, Y. Han, Z. Yuan, Q. Weng, Testicular expression of NGF, TrkA and p75 during seasonal spermatogenesis of the wild ground squirrel (Citellus dauricus Brandt) , European Journal of Histochemistry: Vol. 59 No. 3 (2015)
- Yu Wang, Ziyi Wang, Wenyang Yu, Xia Sheng, Haolin Zhang, Yingying Han, Zhengrong Yuan, Qiang Weng, Seasonal expressions of androgen receptor, estrogen receptors and cytochrome P450 aromatase in the uteri of the wild Daurian ground squirrels (Spermophilus dauricus) , European Journal of Histochemistry: Vol. 62 No. 1 (2018)
- L. Bao, Q. Li, Y. Liu, B. Li, X. Sheng, Y. Han, Q. Weng, Immunolocalization of NGF and its receptors in ovarian surface epithelium of the wild ground squirrel during the breeding and nonbreeding seasons , European Journal of Histochemistry: Vol. 58 No. 2 (2014)
- F. Zhang, J. Wang, Y. Jiao, L. Zhang, H. Zhang, X. Sheng, Y. Han, Z. Yuan, Q. Weng, Seasonal changes of androgen receptor, estrogen receptors and aromatase expression in the medial preoptic area of the wild male ground squirrels (Citellus dauricus Brandt) , European Journal of Histochemistry: Vol. 60 No. 2 (2016)
- Q. Li, F. Zhang, S. Zhang, X. Sheng, X. Han, Q. Weng, Z. Yuan, Seasonal expression of androgen receptor, aromatase, and estrogen receptor alpha and beta in the testis of the wild ground squirrel (Citellus dauricus Brandt) , European Journal of Histochemistry: Vol. 59 No. 1 (2015)
- Qingjing Gao, Wenqian Xie, Wenjing Lu, Yuning Liu, Haolin Zhang, Yingying Han, Qiang Weng, Seasonal patterns of prolactin, prolactin receptor, and STAT5 expression in the ovaries of wild ground squirrels (Citellus dauricus Brandt) , European Journal of Histochemistry: Vol. 67 No. 4 (2023)
- Zeqi Tang, Xiaojie Yuan, Yuming Bai, Yiming Guo, Haolin Zhang, Yingying Han, Zhengrong Yuan, Qiang Weng, Seasonal changes in the expression of PACAP, VPAC1, VPAC2, PAC1 and testicular activity in the testis of the muskrat (Ondatra zibethicus) , European Journal of Histochemistry: Vol. 66 No. 2 (2022)
- Yankun Wang, Yuning Liu, Yawei Wang, Ao Zhang, Wenqian Xie, Haolin Zhang, Qiang Weng, Meiyu Xu, Investigation of seasonal changes in lipid synthesis and metabolism-related genes in the oviduct of Chinese brown frog (Rana dybowskii) , European Journal of Histochemistry: Vol. 67 No. 4 (2023)
- Y. Liu, J. Weng, S. Huang, Y. Shen, X. Sheng, Y. Han, M. Xu, Q. Weng, Immunoreactivities of PPARγ2, leptin and leptin receptor in oviduct of Chinese brown frog during breeding period and pre-hibernation , European Journal of Histochemistry: Vol. 58 No. 3 (2014)
- Liqin Xi, Chen Wang, Pengyu Chen, Qi Yang, Ruiqi Hu, Haolin Zhang, Qiang Weng, Meiyu Xu, Expressions of IL-6, TNF-α and NF-κB in the skin of Chinese brown frog (Rana dybowskii) , European Journal of Histochemistry: Vol. 61 No. 4 (2017)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2022.3351
https://doi.org/10.4081/ejh.2022.3351