Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
MALAT1 regulates hypertrophy of cardiomyocytes by modulating the miR-181a/HMGB2 pathway
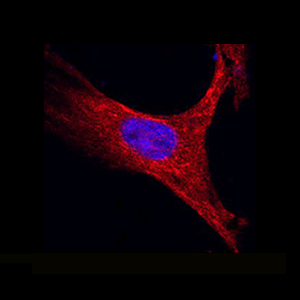
Noncoding RNAs are important for regulation of cardiac hypertrophy. The function of MALAT1 (a long noncoding mRNA), miR-181a, and HMGB2; their contribution to cardiac hypertrophy; and the regulatory relationship between them during this process remain unknown. In the present study, we treated primary cardiomyocytes with angiotensin II (Ang II) to mimic cardiac hypertrophy. MALAT1 expression was significantly downregulated in Ang II-treated cardiomyocytes compared with control cardiomyocytes. Ang II-induced cardiac hypertrophy was suppressed by overexpression of MALAT1 and promoted by genetic knockdown of MALAT1. A dual-luciferase reporter assay demonstrated that MALAT1 acted as a sponge for miR-181a and inhibited its expression during cardiac hypertrophy. Cardiac hypertrophy was suppressed by overexpression of a miR-181a inhibitor and enhanced by overexpression of a miR-181a mimic. HMGB2 was downregulated during cardiac hypertrophy and was identified as a target of miR-181a by bioinformatics analysis and a dual-luciferase reporter assay. miR-181a overexpression decreased the mRNA and protein levels of HMGB2. Rescue experiments indicated that MALAT1 overexpression reversed the effect of miR-181a on HMGB2 expression. In summary, the results of the present study show that MALAT1 acts as a sponge for miR-181a and thereby regulates expression of HMGB2 and development of cardiac hypertrophy. The novel MALAT1/miR-181a/HMGB2 axis might play a crucial role in cardiac hypertrophy and serve as a new therapeutic target.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
Citations
10.3390/cells14020125
10.2139/ssrn.4598063
10.3390/jcdd10040166
10.1016/j.biopha.2023.115118
10.3389/fendo.2022.1054827
10.3390/life13041011
Ethics Approval
All animal procedures were approved by the Animal Research Committee of Ganzhou People’s Hospital (approval No. SJTY(E) 2018-067)Rights
Science and Technology Project of Jiangxi Province (No. 20203BBGL73188) -- Natural Science Foundation of Jiangxi, China (No. 20212ACB206031)How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3426
https://doi.org/10.4081/ejh.2022.3426