Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Imaging and spectral analysis of autofluorescence patterns in larval head structures of mosquito vectors
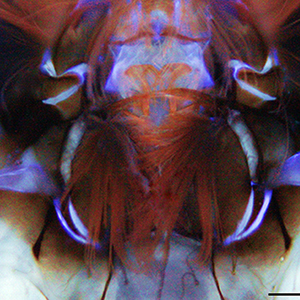
Autofluorescence (AF) in mosquitoes is currently poorly explored, despite its great potential as a marker of body structures and biological functions. Here, for the first time AF in larval heads of two mosquitoes of key public health importance, Aedes albopictus and Culex pipiens, is studied using fluorescence imaging and spectrofluorometry, similarly to a label-free histochemical approach. In generally conserved distribution patterns, AF shows differences between mouth brushes and antennae of the two species. The blue AF ascribable to resilin at the antennal bases, more extended in Cx. pipiens, suggests a potential need to support different antennal movements. The AF spectra larger in Cx. pipiens indicate a variability in material composition and properties likely relatable to mosquito biology, including diverse feeding and locomotion behaviours with implications for vector control.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.