Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Overexpression of hsa_circ_0006470 inhibits the malignant behavior of gastric cancer cells via regulation of miR-1234/TP53I11 axis
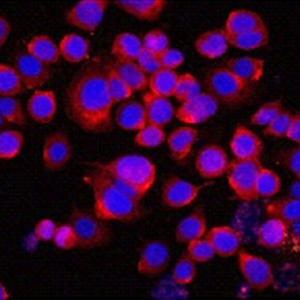
Gastric cancer (GC) is a subtype of a common malignant tumor found in the digestive system. Hsa_circ_0006470 is known to be closely associated with the development of GC. Nevertheless, the mechanism by which hsa_circ_0006470 regulates the tumorigenesis of GC has not been fully elucidated. To investigate the role of hsa_circ_0006470 in GC, its expression levels were assessed in GES-1, AGS, MKN45, and SNU5 cells by reverse transcription-quantitative PCR. Fluorescence in situ hybridization was used to evaluate the localization of hsa_circ_0006470 in AGS and MKN45 cells. In addition, cell counting kit-8 and 5-ethynyl-2’-deoxyuridine assays were performed to evaluate the viability and proliferation of GC cells, respectively. The dual-luciferase reporter assay was used to explore the interaction among hsa_circ_0006470, microRNA (miR)-1234, and TP53I11. The expression levels of TP53I11, Akt, p-Akt, forkhead box O1, and cyclin dependent kinase 2 in AGS cells were analyzed by Western blotting. The data indicated that hsa_circ_0006470 expression was downregulated in AGS cells. In addition, overexpression (OE) of hsa_circ_0006470 could inhibit the viability and proliferation of GC cells. Moreover, OE of hsa_circ_0006470 inhibited the migration of GC cells and induced G1 cell cycle phase arrest. Moreover, miR-1234 was bound to hsa_circ_0006470 and TP53I11 was targeted by miR-1234. Furthermore, OE of hsa_circ_0006470 inhibited the tumorigenesis of GC via the regulation of the miR-1234/TP53I11 axis. In summary, the present study demonstrated that OE of hsa_circ_0006470 notably inhibited the tumorigenesis of GC by regulating the miR-1234/TP53I11 axis. Therefore, the present study may provide a theoretical basis for exploring novel therapeutic strategies for the treatment of GC.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
Ethics Approval
The Ethical Committee of Shanghai Jiading District Central Hospital approved this study (No. SHJDCH20220407)Supporting Agencies
This work was supported by Project of Jiading District Science and Technology Commission (JDKW- 2019-w01)How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2022.3477
https://doi.org/10.4081/ejh.2022.3477