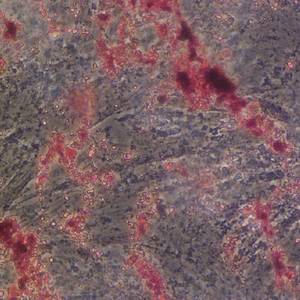
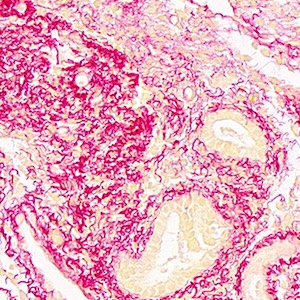
Therapeutic effects of human umbilical cord mesenchymal stem cell-derived extracellular vesicles on ovarian functions through the PI3K/Akt cascade in mice with premature ovarian failure
Submitted: 28 July 2022
Accepted: 28 December 2022
Published: 27 July 2023
Accepted: 28 December 2022
Abstract Views: 930
PDF: 652
HTML: 13
HTML: 13
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Yan Wang, Jie Sun, Ninghua Yao, Correlation of the AKT/mTOR signaling pathway with the clinicopathological features and prognosis of nasopharyngeal carcinoma , European Journal of Histochemistry: Vol. 65 No. 4 (2021)
- D. Fanni, N. Iacovidou, A. Locci, C. Gerosa, S. Nemolato, P. Van Eyken, G. Monga, S. Mellou, G. Faa, V. Fanos, MUC1 marks collecting tubules, renal vesicles, comma- and S-shaped bodies in human developing kidney , European Journal of Histochemistry: Vol. 56 No. 4 (2012)
- Claudia Frick, Hanna Luisa Martin, Johanna Bruder, Kerstin Lang, Heinz Breer, Topographic distribution pattern of morphologically different G cells in the murine antral mucosa , European Journal of Histochemistry: Vol. 61 No. 3 (2017)
- Tadashi Yasui, Kenya Miyata, Chie Nakatsuka, Azuma Tsukise, Hiroshi Gomi, Morphological and histochemical characterization of the secretory epithelium in the canine lacrimal gland , European Journal of Histochemistry: Vol. 65 No. 4 (2021)
- Alimu Keremu, Pazila Aila, Aikebaier Tusun , Maimaitiaili Abulikemu, Xiaoguang Zou, Extracellular vesicles from bone mesenchymal stem cells transport microRNA-206 into osteosarcoma cells and target NRSN2 to block the ERK1/2-Bcl-xL signaling pathway , European Journal of Histochemistry: Vol. 66 No. 3 (2022)
- S. Huang, S. Guo, F. Guo, Q. Yang, X. Xiao, M. Murata, S. Ohnishi, S. Kawanishi, N. Ma, CD44v6 expression in human skin keratinocytes as a possible mechanism for carcinogenesis associated with chronic arsenic exposure , European Journal of Histochemistry: Vol. 57 No. 1 (2013)
- A. Porzionato, M. Rucinski, V. Macchi, G. Sarasin, L.K. Malendowicz, R. De Caro, ECRG4 expression in normal rat tissues: expression study and literature review , European Journal of Histochemistry: Vol. 59 No. 2 (2015)
- G. Chene, N. Radosevic-Robin, A.S. Tardieu, A. Cayre, I. Raoelfils, P. Dechelotte, J. Dauplat, F. Penault Llorca, Morphological and immunohistochemical study of ovarian and tubal dysplasia associated with tamoxifen , European Journal of Histochemistry: Vol. 58 No. 2 (2014)
- R.J. BuÅ‚dak, M. Skonieczna, Å. BuÅ‚dak, N. Matysiak, Å. MielaÅ„czyk, G. Wyrobiec, M. Kukla, M. Michalski, K. Å»wirska-Korczala, Changes in subcellular localization of visfatin in human colorectal HCT-116 carcinoma cell line after cytochalasin B treatment , European Journal of Histochemistry: Vol. 58 No. 3 (2014)
- M. Riccio, E. Resca, T. Maraldi, A. Pisciotta, A. Ferrari, G. Bruzzesi, A. De Pol, Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures , European Journal of Histochemistry: Vol. 54 No. 4 (2010)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2023.3506
https://doi.org/10.4081/ejh.2023.3506