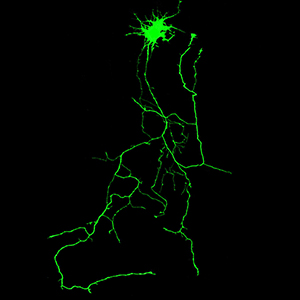
Phosphorylation mutation impairs the promoting effect of spastin on neurite outgrowth without affecting its microtubule severing ability
Submitted: 4 November 2022
Accepted: 27 December 2022
Published: 12 January 2023
Accepted: 27 December 2022
Abstract Views: 947
PDF: 573
HTML: 28
HTML: 28
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- L. Ragionieri, M. Botti, F. Gazza, C. Sorteni, R. Chiocchetti, P. Clavenzani, L. Bo, R. Panu, Localization of peripheral autonomic neurons innervating the boar urinary bladder trigone and neurochemical features of the sympathetic component , European Journal of Histochemistry: Vol. 57 No. 2 (2013)
- Jianyu Zou, Zhenbin Cai, Zhi Liang , Yaozhong Liang, Guowei Zhang, Jie Yang, Yunlong Zhang, Hongsheng Lin, Minghui Tan, Different fusion tags affect the activity of ubiquitin overexpression on spastin protein stability , European Journal of Histochemistry: Vol. 65 No. 4 (2021)
- Valentina Alda Carozzi, Chiara Salio, Virginia Rodriguez-Menendez, Elisa Ciglieri, Francesco Ferrini, 2D vs 3D morphological analysis of dorsal root ganglia in health and painful neuropathy , European Journal of Histochemistry: Vol. 65 No. s1 (2021): Special Collection on Advances in Neuromorphology in Health and Disease
- J.P. Damico, E. Ervolino, K.R. Torres, D.S. Batagello, R.J. Cruz-Rizzolo, C.A. Casatti, J.A. Bauer, Phenotypic alterations of neuropeptide Y and calcitonin gene-related peptide-containing neurons innervating the rat temporomandibular joint during carrageenan-induced arthritis , European Journal of Histochemistry: Vol. 56 No. 3 (2012)
- Andrea Conz, Clara Alice Musi, Luca Russo, Tiziana Borsello, Luca Colnaghi, Super-resolution study of PIAS SUMO E3-ligases in hippocampal and cortical neurons , European Journal of Histochemistry: Vol. 65 No. s1 (2021): Special Collection on Advances in Neuromorphology in Health and Disease
- W. Romero-Fernandez, D.O. Borroto-Escuela, V. Vargas-Barroso, M. Narváez, M. Di Palma, L.F. Agnati, J. Larriva Sahd, K. Fuxe, Dopamine D1 and D2 receptor immunoreactivities in the arcuate-median eminence complex and their link to the tubero-infundibular dopamine neurons , European Journal of Histochemistry: Vol. 58 No. 3 (2014)
- A. Bolekova, T. Spakovska, D. Kluchova, S. Toth, J. Vesela, NADPH-diaphorase expression in the rat jejunum after intestinal ischemia/reperfusion , European Journal of Histochemistry: Vol. 55 No. 3 (2011)
- S. Salucci, P. Ambrogini, D. Lattanzi, M. Betti, P. Gobbi, C. Galati, F. Galli, R. Cuppini, A. Minelli, Maternal dietary loads of alpha-tocopherol increase synapse density and glial synaptic coverage in the hippocampus of adult offspring , European Journal of Histochemistry: Vol. 58 No. 2 (2014)
- M. Ferreira Júnior, S.A. Batista, P.V.T. Vidigal, A.A.C. Cordeiro, F.M.S. Oliveira, L.O. Prata, A.E.T. Diniz, C.M. Barral, R.C. Barbuto, A.D. Gomes, I.D. Araújo, D.M.M. Queiroz, M.V. Caliari, Infection with CagA-positive Helicobacter pylori strain containing three EPIYA C phosphorylation sites is associated with more severe gastric lesions in experimentally infected Mongolian gerbils (Meriones unguiculatus) , European Journal of Histochemistry: Vol. 59 No. 2 (2015)
- W.J. Liu, J. Yang, Preferentially regulated expression of connexin 43 in the developing spiral ganglion neurons and afferent terminals in post-natal rat cochlea , European Journal of Histochemistry: Vol. 59 No. 1 (2015)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2023.3594
https://doi.org/10.4081/ejh.2023.3594











