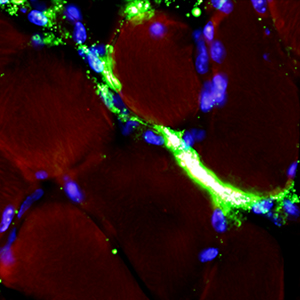
An ex vivo experimental system to track fluorescent nanoparticles inside skeletal muscle
Submitted: 7 November 2022
Accepted: 14 December 2022
Published: 22 December 2022
Accepted: 14 December 2022
Abstract Views: 687
PDF: 441
HTML: 28
HTML: 28
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Jinshuang Li, Dawei Xu, Ce Shi, Chunqi Cheng, Ziheng Xu, Xingjuan Gao, Yong Cheng, Alarin regulates RyR2 and SERCA2 to improve cardiac function in heart failure with preserved ejection fraction , European Journal of Histochemistry: Vol. 68 No. 4 (2024)
- Roberta Sferra, Simona Pompili, Claudio Festuccia, Francesco Marampon, Giovanni L. Gravina, Luca Ventura, Ernesto Di Cesare, Sara Cicchinelli, Eugenio Gaudio, Antonella Vetuschi, The possible prognostic role of histone deacetylase and transforming growth factor β/Smad signaling in high grade gliomas treated by radio-chemotherapy: a preliminary immunohistochemical study , European Journal of Histochemistry: Vol. 61 No. 2 (2017)
- Yanmei Yao, Leqing Lin, Wenxue Tang, Yueliang Shen, Fayu Chen, Ning Li, Baiyong Wang, Pretreatment with geniposide mitigates myocardial ischemia/reperfusion injury by modulating inflammatory response through TLR4/NF-κB pathway , European Journal of Histochemistry: Vol. 67 No. 3 (2023)
- I. Ferrandino, R. Favorito, M. C. Grimaldi, Cadmium induces changes on ACTH and PRL cells in Podarcis sicula Lizard pituitary gland , European Journal of Histochemistry: Vol. 54 No. 4 (2010)
- A. Di Vito, E. Scali, G. Ferraro, C. Mignogna, I. Presta, C. Camastra, C. Palmieri, G. Donato, T. Barni, Elastofibroma dorsi: a histochemical and immunohistochemical study , European Journal of Histochemistry: Vol. 59 No. 1 (2015)
- A.M. Schläfli, S. Berezowska, O. Adams, R. Langer, M.P. Tschan, Reliable LC3 and p62 autophagy marker detection in formalin fixed paraffin embedded human tissue by immunohistochemistry , European Journal of Histochemistry: Vol. 59 No. 2 (2015)
- M. Onisto, S. Garbisa, M. Spina, Lorenzo Gotte (1926-1991): a pioneer of elastin , European Journal of Histochemistry: Vol. 60 No. 3 (2016)
- Nuobei Zhang, Hao Shen, Shenan Huang, Fenfen Wang, Huifang Liu, Fen Xie, Lei Jiang, Xin Chen, LncRNA FGD5-AS1 functions as an oncogene to upregulate GTPBP4 expression by sponging miR-873-5p in hepatocellular carcinoma , European Journal of Histochemistry: Vol. 65 No. 4 (2021)
- S. Salucci, S. Burattini, E. Falcieri, P. Gobbi, Three-dimensional apoptotic nuclear behavior analyzed by means of Field Emission in Lens Scanning Electron Microscope , European Journal of Histochemistry: Vol. 59 No. 3 (2015)
- Barbara Lanza, Anna Panato, Laura Valentini, Pamela Rodegher, Federica Bortolotti, Michela Battistelli, Paolino Ninfali, Pietro Gobbi, A morphological analysis of fresh and brine-cured olives attacked by Bactrocera oleae using light microscopy and ESEM-EDS , European Journal of Histochemistry: Vol. 64 No. 3 (2020)
<< < 24 25 26 27 28 29 30 31 32 33 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2023.3596
https://doi.org/10.4081/ejh.2023.3596











