Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Nrf2 as a novel diagnostic biomarker for papillary thyroid carcinoma
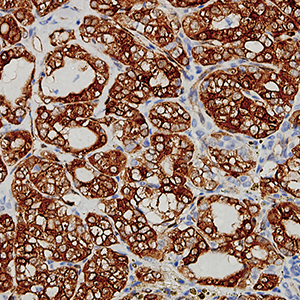
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy. However, it is very difficult to distinguish PTC from benign carcinoma. Thus, specific diagnostic biomarkers are actively pursued. Previous studies observed that Nrf2 was highly expressed in PTC. Based on this research, we hypothesized that Nrf2 may serve as a novel specific diagnostic biomarker. A single-center retrospective study, including 60 patients with PTC and 60 patients with nodular goiter, who underwent thyroidectomy at the Central Theater General Hospital from 2018 to July 2020, was conducted. The clinical data of the patients were collected. Nrf2, BRAF V600E, CK-19, and Gal-3 proteins were compared from paraffin samples of the patients. Through this study, we obtained the following results: i) Nrf2 exhibits high abundance expression in PTC, but not in adjacent to PTC and nodular goiter; increased Nrf2 expression could serve as a valuable biomarker for PTC diagnosis; the sensitivity and specificity for the diagnosis of PTC were 96.70% and 89.40%, respectively. ii) Nrf2 also shows higher expression in PTC with lymph node metastasis, but not adjacent to PTC and nodular goiter, thus the increased Nrf2 expression might serve as a valuable predictor for lymph node metastasis in PTC patients; the sensitivity and specificity for the prediction in lymph node metastasis were 96.00% and 88.57%, respectively; excellent diagnostic agreements were found between Nrf2 and other routine parameters including HO-1, NQO1 and BRAF V600E. iii) The downstream molecular expression of Nrf2 including HO-1 and NQO1 consistently increased. In conclusion, Nrf2 displays a high abundance expression in human PTC, which leads to the higher expression of downstream transcriptional proteins: HO-1 and NQO1. Moreover, Nrf2 can be used as an extra biomarker for differential diagnosis of PTC and a predictive biomarker for lymph node metastasis of PTC.
Altmetrics
Downloads
Edited by
This study was approved by the Research Ethics Committee of the General Hospital of Central Theater Command, Wuhan, Hubei, China (N. [2021]005-02)Supporting Agencies
Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology, China International Medical FoundationHow to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.