Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Developmental expression of high-mobility group box 1 (HMGB1) in the mouse cochlea
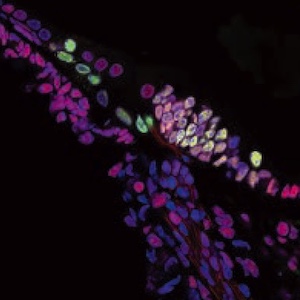
The expression changes of high-mobility group box 1 (HMGB1) in the mouse cochlea have recently been implicated in noise-induced hearing loss, suggesting that HMGB1 participates in regulating cochlear function. However, the precise role of HMGB1 in the auditory system remains largely unclear. This study aimed to investigate its function in the developing mouse cochlea by examining the expression pattern of HMGB1 in the mouse cochlea from embryonic day (E) 18.5 to postnatal day (P) 28 using double immunofluorescence on frozen sections. Our findings revealed that HMGB1 was extensively expressed in the cell nucleus across various regions of the mouse cochlea, including the organ of Corti. Furthermore, its expression underwent developmental regulation during mouse cochlear development. Specifically, HMGB1 was found to be localized in the tympanic border cells at each developmental stage, coinciding with the gradual anatomical in this region during development. In addition, HMGB1 was expressed in the greater epithelial ridge (GER) and supporting cells of the organ of Corti, as validated by the supporting cell marker Sox2 at P1 and P8. However, at P14, the expression of HMGB1 disappeared from the GER, coinciding with the degeneration of the GER into the inner sulcus cells. Moreover, we observed that HMGB1 co-localized with Ki-67-positive proliferating cells in several cochlear regions during late embryonic and early postnatal stages, including the GER, the tympanic border cells, cochlear lateral wall, and cochlear nerves. Furthermore, by dual-staining Ki-67 with neuronal marker TUJ1 and glial marker Sox10, we determined the expression of Ki-67 in the neonatal glial cells. Our spatial-temporal analysis demonstrated that HMGB1 exhibited distinct expression patterns during mouse cochlear development. The co-localization of HMGB1 with Ki-67-positive proliferating cells suggested that HMGB1 may play a role in cochlear development.
Downloads
Publication Facts
Reviewer profiles N/A
Author statements
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy
Citations
10.4081/ejh.2025.4189
10.4081/ejh.2024.4137
Supporting Agencies
National Natural Science Foundation of China, Natural Science Foundation of Jiangsu ProvinceHow to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejh.2023.3704
https://doi.org/10.4081/ejh.2023.3704