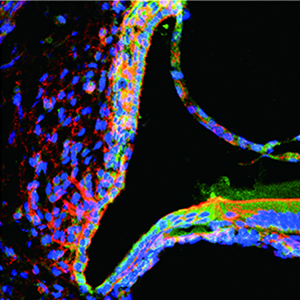
Expression and localization of α2A-adrenergic receptor in the rat post-natal developing cochlea
Submitted: 11 April 2023
Accepted: 22 July 2023
Published: 7 August 2023
Accepted: 22 July 2023
Abstract Views: 475
PDF: 393
Supplementary: 47
HTML: 7
Supplementary: 47
HTML: 7
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- G Pons, V Evangelisti, F Caprì, S Mozzone, A Viarengo, Cytochemical localization and quantification of plasma membrane Ca2+-ATPase activity in mollusc digestive gland cells , European Journal of Histochemistry: Vol. 46 No. 1 (2002)
- Carlo Alberto Redi, Transgenic mouse - Methods and protocols, 2nd edition , European Journal of Histochemistry: Vol. 55 No. 3 (2011)
- Daniëlle Krijgsman, Ronald L.P. van Vlierberghe, Vaios Evangelou, Alexander L. Vahrmeijer, Cornelis J.H. van de Velde, Cornelis F.M. Sier, Peter J.K. Kuppen, A method for semi-automated image analysis of HLA class I tumour epithelium expression in rectal cancer , European Journal of Histochemistry: Vol. 63 No. 2 (2019)
- M Lehnert, H Laurer, B Maier, J Frank, I Marzi, W-I Steudel, A Mautes, The histochemical profile of the rat extensor digitorum longus muscle differentiates after birth and dedifferentiates in senescence , European Journal of Histochemistry: Vol. 51 No. 2 (2007)
- D. Fanni, S. Nemolato, R. Ganga, G. Senes, C. Gerosa, P. Van Eyken, K. Geboes, G. Faa, Cytokeratin 20-positive hepatocellular carcinoma , European Journal of Histochemistry: Vol. 53 No. 4 (2009)
- E Sanna, MP Sanna, C Loddo, Endogenous Jaagsiekte Sheep Retrovirus RNA is expressed by different cell types in ovine foetus and placenta , European Journal of Histochemistry: Vol. 46 No. 3 (2002)
- V Nicolin, C Ponti, G Baldini, D Gibellini, R Bortul, M Zweyer, B Martinelli, P Narducci, In vitro exposure of human chondrocytes to pulsed electromagnetic fields , European Journal of Histochemistry: Vol. 51 No. 3 (2007)
- C. Soldani, M.G. Bottone, M. Biggiogera, C. Alpini, A.I. Scovassi, T. Martin, C. Pellicciari, Nuclear localization of phosphorylated c-Myc protein in human tumor cells. , European Journal of Histochemistry: Vol. 46 No. 4 (2002)
- Carlo Alberto Redi, RNA therapeutics - Function, design and delivery , European Journal of Histochemistry: Vol. 55 No. 2 (2011)
- A.M. Luciano, V. Lodde, F. Franciosi, I. Tessaro, D. Corbani, S. Modina, Large-scale chromatin morpho-functional changes during mammalian oocyte growth and differentiation , European Journal of Histochemistry: Vol. 56 No. 3 (2012)
<< < 68 69 70 71 72 73 74 75 76 77 > >>
You may also start an advanced similarity search for this article.
Publication Facts
Metric
This article
Other articles
Peer reviewers
2
2.4
Reviewer profiles N/A
Author statements
Author statements
This article
Other articles
Data availability
N/A
16%
External funding
N/A
32%
Competing interests
N/A
11%
Metric
This journal
Other journals
Articles accepted
57%
33%
Days to publication
117
145
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy

 https://doi.org/10.4081/ejh.2023.3748
https://doi.org/10.4081/ejh.2023.3748












