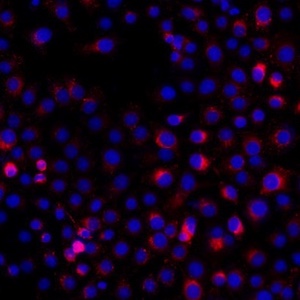
Tumor cells-derived exosomal PD-L1 promotes the growth and invasion of lung cancer cells in vitro via mediating macrophages M2 polarization
Submitted: 27 May 2023
Accepted: 3 July 2023
Published: 1 August 2023
Accepted: 3 July 2023
Abstract Views: 739
PDF: 519
HTML: 10
HTML: 10
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- A. Gonelli, D. Milani, E. Rimondi, R. Voltan, V. Grill, C. Celeghini, Activation of PKC-e counteracts maturation and apoptosis of HL-60 myeloid leukemic cells in response to TNF family members , European Journal of Histochemistry: Vol. 53 No. 3 (2009)
- Zhao Zhang, Hongming Fan, William Richardson, Bruce Z. Gao, Tong Ye, Management of autofluorescence in formaldehyde-fixed myocardium: choosing the right treatment , European Journal of Histochemistry: Vol. 67 No. 4 (2023)
- Bin Ma, Changfu Yin, Dailun Hu, Mark Newman, Philip K. Nicholls, Zhanjun Wu, Wayne K. Greene, Zhongli Shi, Distribution of non-myelinating Schwann cells and their associations with leukocytes in mouse spleen revealed by immunofluorescence staining , European Journal of Histochemistry: Vol. 62 No. 2 (2018)
- S Matsubara, T Kato, K Oshikawa, T Yamada, T Takayama, T Koike, T Watanabe, A Izumi, I Sato, Glucose-6-phosphate dehydrogenase in rat lung alveolar epithelial cells. An ultrastructural enzyme-cytochemical study , European Journal of Histochemistry: Vol. 46 No. 3 (2002)
- CarloAlberto Redi, Light microscopy - Methods and protocols , European Journal of Histochemistry: Vol. 55 No. 4 (2011)
- Carlo Alberto Redi, DNA damage detection In situ, ex vivo and In vivo - Methods and protocols , European Journal of Histochemistry: Vol. 55 No. 2 (2011)
- Carlo Alberto Redi, A picture is worth a thousand tables - graphics in life sciences , European Journal of Histochemistry: Vol. 57 No. 3 (2013)
- L. Benerini Gatta, M. Cadei, P. Balzarini, S. Castriciano, R. Paroni, A. Verzeletti, V. Cortellini, F. De Ferrari, P. Grigolato, Application of alternative fixatives to formalin in diagnostic pathology , European Journal of Histochemistry: Vol. 56 No. 2 (2012)
- Carlo Alberto Redi, Regenerative medicine and cell therapy , European Journal of Histochemistry: Vol. 57 No. 2 (2013)
- CarloAlberto Redi, Adult stem cells - Biology and methods of analysis , European Journal of Histochemistry: Vol. 55 No. 4 (2011)
<< < 49 50 51 52 53 54 55 56 57 58 > >>
You may also start an advanced similarity search for this article.
Publication Facts
Metric
This article
Other articles
Peer reviewers
2
2.4
Reviewer profiles N/A
Author statements
Author statements
This article
Other articles
Data availability
N/A
16%
External funding
N/A
32%
Competing interests
N/A
11%
Metric
This journal
Other journals
Articles accepted
57%
33%
Days to publication
65
145
- Academic society
- N/A
- Publisher
- PAGEPress Publications, Pavia, Italy

 https://doi.org/10.4081/ejh.2023.3784
https://doi.org/10.4081/ejh.2023.3784












