Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Ozone and procaine increase secretion of platelet-derived factors in platelet-rich plasma
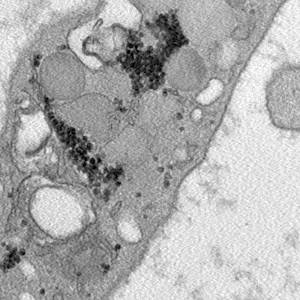
Platelet-rich plasma (PRP) is gaining more and more attention in regenerative medicine as an innovative and efficient therapeutic approach. The regenerative properties of PRP rely on the numerous bioactive molecules released by the platelets: growth factors are involved in proliferation and differentiation of endothelial cells and fibroblasts, angiogenesis and extracellular matrix formation, while cytokines are mainly involved in immune cell recruitment and inflammation modulation. Attempts are ongoing to improve the therapeutic potential of PRP by combining it with agents able to promote regenerative processes. Two interesting candidates are ozone, administered at low doses as gaseous oxygen-ozone mixtures, and procaine. In the present study, we investigated the effects induced on platelets by the in vitro treatment of PRP with ozone or procaine, or both. We combined transmission electron microscopy to obtain information on platelet modifications and bioanalytical assays to quantify the secreted factors. The results demonstrate that, although platelets were already activated by the procedure to prepare PRP, both ozone and procaine induced differential morpho-functional modifications in platelets resulting in an increased release of factors. In detail, ozone induced an increase in surface protrusions and open canalicular system dilation suggestive of a marked α-granule release, while procaine caused a decrease in surface protrusions and open canalicular system dilation but a remarkable increase in microvesicle release suggestive of high secretory activity. Consistently, nine of the thirteen platelet-derived factors analysed in the PRP serum significantly increased after treatment with ozone and/or procaine. Therefore, ozone and procaine proved to have a remarkable stimulating potential without causing any damage to platelets, probably because they act through physiological, although different, secretory pathways.
Altmetrics
Downloads
Edited by
this study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. The written informed consent was obtained from volunteer donors.How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.