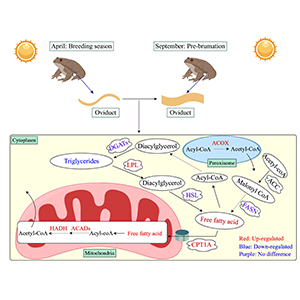
Investigation of seasonal changes in lipid synthesis and metabolism-related genes in the oviduct of Chinese brown frog (Rana dybowskii)
Submitted: 17 October 2023
Accepted: 9 December 2023
Published: 20 December 2023
Accepted: 9 December 2023
Abstract Views: 519
PDF: 292
HTML: 8
HTML: 8
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Bo Dai, Hailin Liu, Dingmin Juan, Kaize Wu, Ruhao Cao, The role of miRNA-29b1 on the hypoxia-induced apoptosis in mammalian cardiomyocytes , European Journal of Histochemistry: Vol. 68 No. 3 (2024)
- M. Riccio, E. Resca, T. Maraldi, A. Pisciotta, A. Ferrari, G. Bruzzesi, A. De Pol, Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures , European Journal of Histochemistry: Vol. 54 No. 4 (2010)
- C. Fede, G. Albertin, L. Petrelli, M.M. Sfriso, C. Biz, R. De Caro, C. Stecco, Expression of the endocannabinoid receptors in human fascial tissue , European Journal of Histochemistry: Vol. 60 No. 2 (2016)
- Zeqi Tang, Xiaojie Yuan, Yuming Bai, Yiming Guo, Haolin Zhang, Yingying Han, Zhengrong Yuan, Qiang Weng, Seasonal changes in the expression of PACAP, VPAC1, VPAC2, PAC1 and testicular activity in the testis of the muskrat (Ondatra zibethicus) , European Journal of Histochemistry: Vol. 66 No. 2 (2022)
- Yong Jiang, Chun-hui Xu, Ying Zhao, Yun-han Ji, Xin-tao Wang, Ying Liu, LINC00926 is involved in hypoxia-induced vascular endothelial cell dysfunction via miR-3194-5p regulating JAK1/STAT3 signaling pathway , European Journal of Histochemistry: Vol. 67 No. 1 (2023)
- C. Severi, R. Sferra, A. Scirocco, A. Vetuschi, N. Pallotta, A. Pronio, R. Caronna, G. Di Rocco, E. Gaudio, E. Corazziari, P. Onori, Contribution of intestinal smooth muscle to Crohn's disease fibrogenesis , European Journal of Histochemistry: Vol. 58 No. 4 (2014)
- Jianyu Zou, Zhenbin Cai, Zhi Liang , Yaozhong Liang, Guowei Zhang, Jie Yang, Yunlong Zhang, Hongsheng Lin, Minghui Tan, Different fusion tags affect the activity of ubiquitin overexpression on spastin protein stability , European Journal of Histochemistry: Vol. 65 No. 4 (2021)
- I. Kopera, M. Durlej, A. Hejmej, K. Knapczyk-Stwora, M. Duda, M. Slomczynska, M. Koziorowski, B. Bilinska, Effects of pre- and postnatal exposure to flutamide on connexin 43 expression in testes and ovaries of prepubertal pigs , European Journal of Histochemistry: Vol. 54 No. 2 (2010)
- L. Ragionieri, M. Botti, F. Gazza, C. Sorteni, R. Chiocchetti, P. Clavenzani, L. Bo, R. Panu, Localization of peripheral autonomic neurons innervating the boar urinary bladder trigone and neurochemical features of the sympathetic component , European Journal of Histochemistry: Vol. 57 No. 2 (2013)
- M. Onisto, S. Garbisa, M. Spina, Lorenzo Gotte (1926-1991): a pioneer of elastin , European Journal of Histochemistry: Vol. 60 No. 3 (2016)
<< < 16 17 18 19 20 21 22 23 24 25 > >>
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejh.2023.3890
https://doi.org/10.4081/ejh.2023.3890











